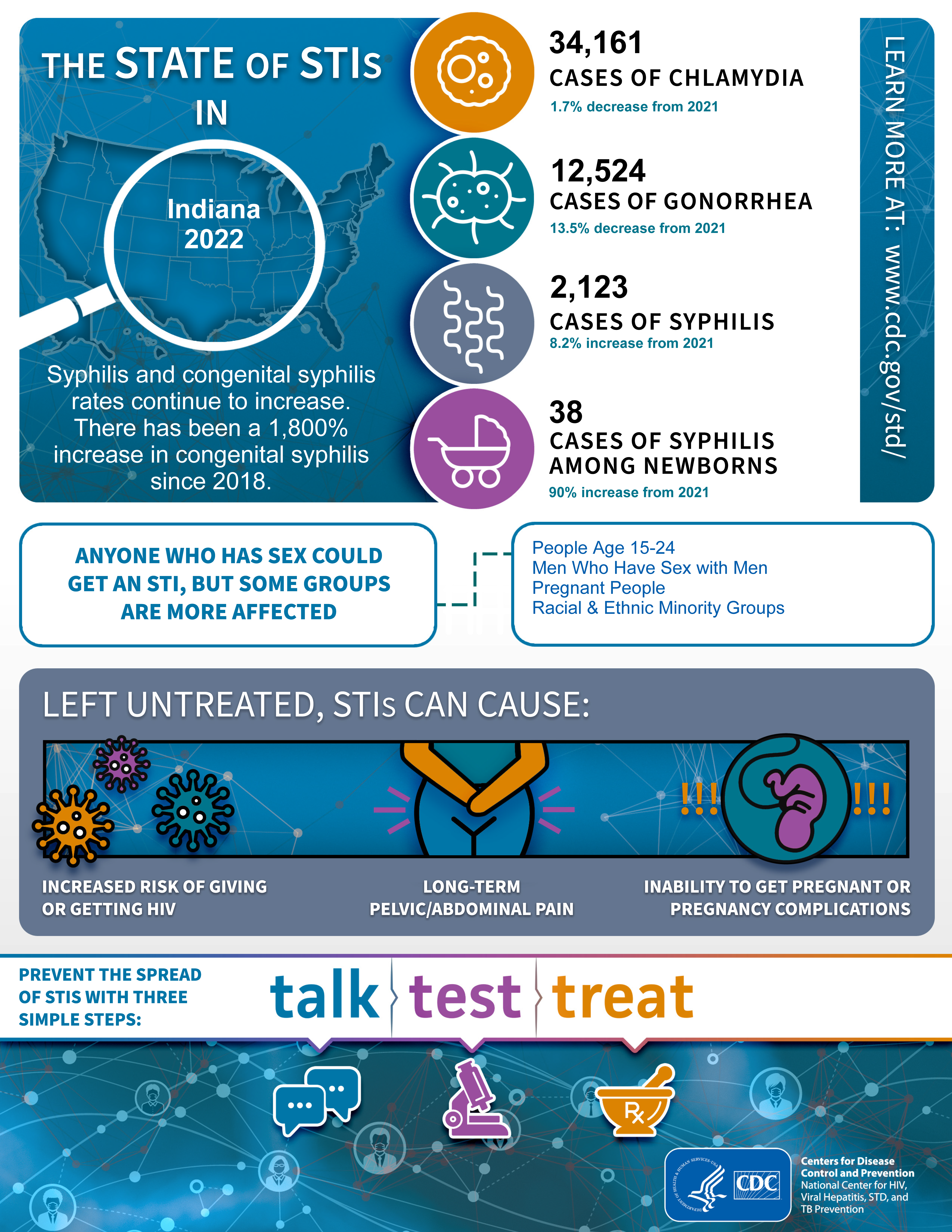
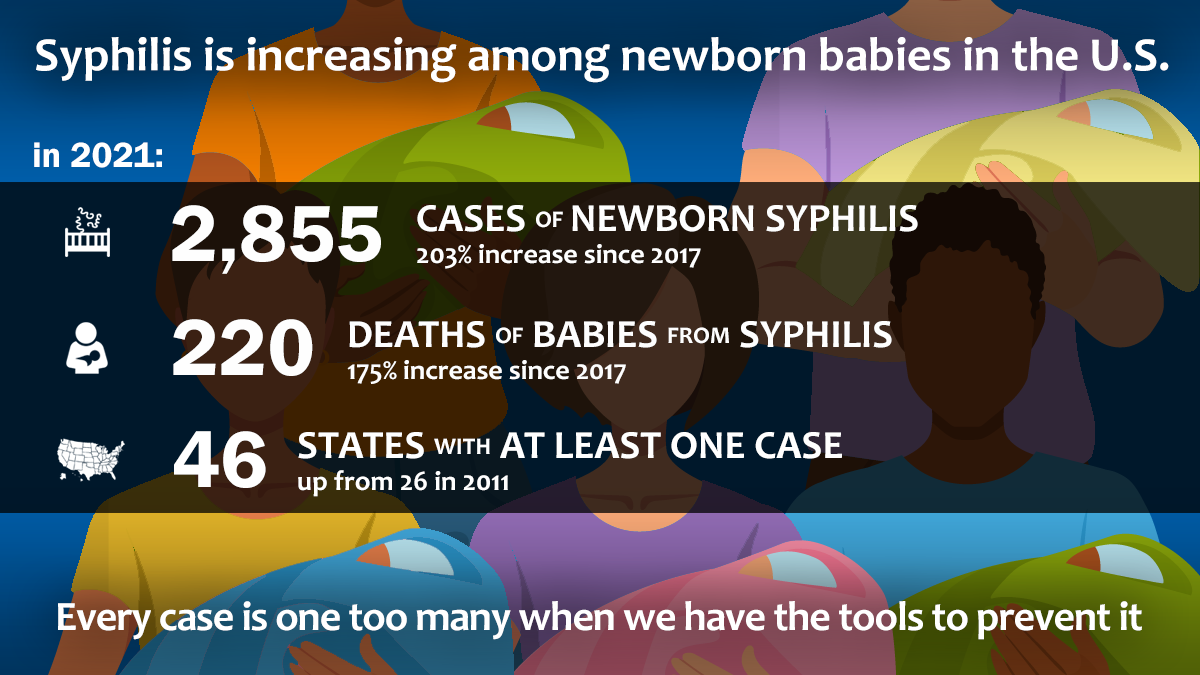
The mission of the STI Prevention Program is to intervene in the spread of sexually transmitted diseases (STIs) and reduce the complications of these diseases. The program provides technical and financial assistance to local STI programs for surveillance, case detection through screening, ensuring treatment of known cases, case follow-up, and education. Efforts are coordinated among health care providers to screen for syphilis, gonorrhea, and chlamydia. Other important aspects of this program include education and prevention counseling for persons impacted by STIs.
The STI Prevention Program seeks to engage community groups, partner agencies, and other stakeholders to decrease the disparity of STIs among vulnerable communities and populations. This includes improving health inequalities that exist among people with STIs, including categorial inequalities defined by the WHO as “demographically, economically, geographically, and socially disadvantaged.”
ALERT: Please review the following guidance regarding the Bicillin L-A Recall.
- Overview and Guidance
Overview:
- On July 10, King Pharmaceuticals LLC. (a subsidy of Pfizer), voluntarily recalled lots of Bicillin L-A (Penicillin G Benzathine Injectable Suspension), due to particulates identified during visual inspection. The impacted timeframe in which affected product lots were distributed was from Dec. 11, 2023, through June 24, 2025. To date, Pfizer has not received reports of any adverse events associated with this issue. Please refer to the above link for lot numbers, as well as product photos and labels for ease of identifying the impacted product.
- Pfizer issued a statement that same day regarding planning for patient care. Pfizer is anticipating a near-term stockout for Bicillin L-A due to this voluntary recall.
- The American Society of Health-System Pharmacists (ASHP) has updated its website with information regarding the products affected, reasons for the shortage, available products, estimated resupply dates, implications for patient care, and alternative agents and management.
The Indiana Department of Health (IDOH) is returning inventory that may be impacted by this voluntary recall. Please email Special Projects Manager Jeremy Roseberry if you have questions about this voluntary recall and anticipated near-term stockout.
What can you do?- Pfizer has provided guidance on how to check your current stock. If you have affected product lots, please discontinue use, stop distribution and quarantine the product immediately. Promptly return the product to Sedgwick; 2670 Executive Drive, Suite A; Indianapolis, IN 46241; Attn: Event 8637, or call Sedgwick at 800-805-3093. Affected product lots.
- Pfizer has implemented its Medical Request Process, effective immediately. The purpose of this process is to ensure that available inventory is distributed equitably to hospitals and clinics treating patients with the highest medical necessity, which, based on prior CDC guidance during Bicillin L-A shortages, is to prioritize product only for patients with confirmed congenital syphilis and risk of congenital syphilis. Please share this guidance with your provider networks.
- FOR IDOH PREVENTION PROGRAM GRANTEES ONLY: Please email Courtney Schaber, Jeremy Roseberry and Silva Tunio if you are running low on medication, and we will do our best to accommodate your needs. Please let us know how much medication you have on hand and your anticipated need, for consideration. Orders may be limited at this time.
Finally, the IDOH Prevention Program asks that providers do the following:
- Continue to follow the Centers for Disease Control and Prevention’s treatment recommendations for sexually-transmitted infections
- Prescribe Bicillin L-A as the only recommended treatment for pregnant women infected with or exposed to syphilis
- Consider prioritizing using Bicillin L-A to treat pregnant women and babies with congenital syphilis, if applicable. All other cases can be treated with doxycycline 100mg PO BID for two weeks (for early syphilis) or four weeks (for latent or syphilis of unknown duration)
Thank you for your continued partnership and dedication to the communities you serve. This page will be updated as we learn new information.